July 10, 2024
BioDigit MG: A Multi-Modal Digital Health Technology for Myasthenia Gravis
I am thrilled to announce the completion of BioDigit MG, our first clinical study in myasthenia gravis (MG) focused on the development and validation of a multi-modal digital health technology for monitoring MG symptoms. Also I am pleased to announce that our work has acknowledged with the 2024 AANEM President’s Research Initiative Award!
In this study, supported by $2.5M of funding from the NIH, we collaborated with Amanda Guidon, MD (Massachusetts General Hospital), and 3 other MG experts to develop the digital health technology for objective, precise and frequent monitoring of disease symptoms in MG. We used the developed tool to collect data from 20 MG patients, and also conducted interviews with stakeholders using the Technical Acceptance Model (TAM) and the Usability Metric for User Experience (UMUX).
The developed technology incorporates several BioSensics products listed below, and is currently available in 11 different languages:
MG-related clinical questionnaires, including MG-ADL and MG-QoL15r
BioDigit Speech for frequent assessment of speech dysfunction and dysarthria
BioDigit Video to precisely detect facial features, specifically targeting eye landmarks, to assess conditions such as ptosis and double vision.
PAMSys and PAMSys ULM wearable sensors for monitoring ambulation, fatigue, and upper limb function.
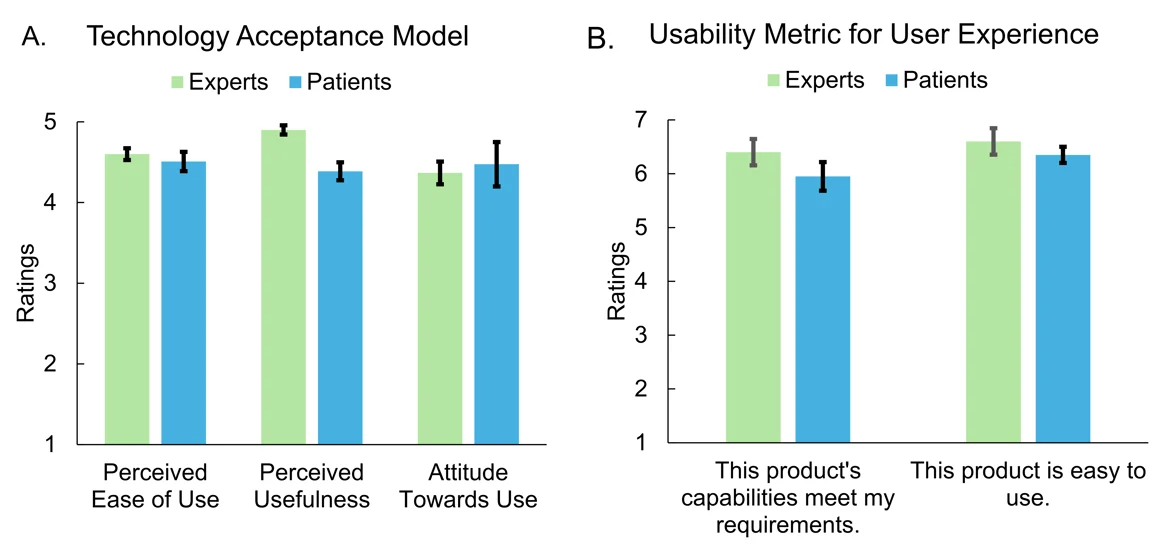
Figure A below shows the average ratings from expert clinicians (n=5) with an average of 21.2 year of practice and MG patients (n=20, average age = 59 ± 3.6 years, with disease duration ranging from 3 months to 29 years) for the three major constructs of the TAM: 1) perceived ease of use, 2) perceived usefulness, and 3) attitude towards use (5 is the maximum score for each construct, where 5 represents a significantly user-friendly rating).
Figure B presents the average ratings for the 2 items of UMUX: 1) This product’s capabilities meet my requirements, and 2) This product is easy to use (7 is the maximum score for each item, signifying optimal functionality and complete fulfillment of user requirements). The results clearly demonstrate that participants find the developed solution not only highly effective and easy to use but also well-suited to their needs.
Later this year, we will start a larger clinical validation study using the developed technology with 50 MG patients who will be followed for 12 months.