May 22, 2024
NIH Selected BioSensics Technology for Rare Disease Clinical Trials
We are excited to share that the National Institutes of Health (NIH) has chosen BioSensics products as the remote measurement technology for clinical trials in rare diseases. NIH has granted BioSensics a $2M contract to enhance and validate its clinical trial products, including BioDigit Home and BioDigit Mobile, for use in trials targeting rare neurological and neuromuscular diseases.
As a part of this contract, we will validate the use of our solutions in three rare neurological disorders in collaboration with Anne-Marie Wills, M.D., (Massachusetts General Hospital) and Alex Pantelyat, M.D. (Johns Hopkins University).
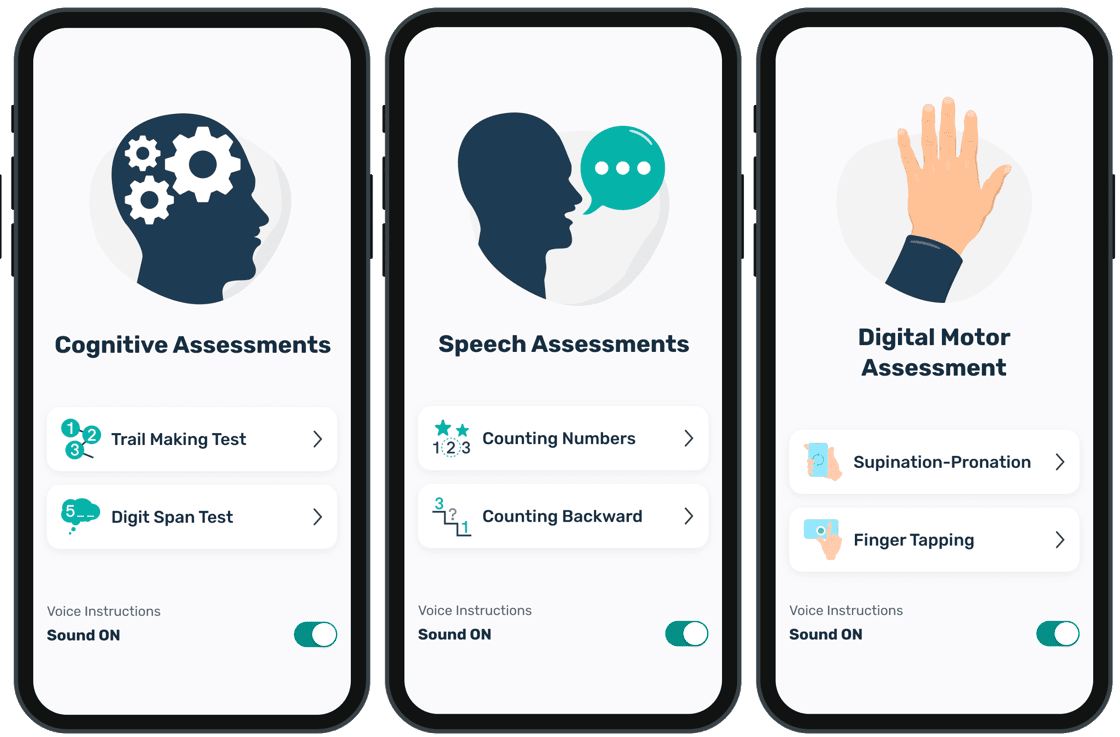
BioDigit Home and BioDigit Mobile are comprehensives solutions for remote collection of PROs, diary entries, digital speech and video-based assessments, and wearable sensor data. These products streamline the integration of the PAMSys sensor for precision actigraphy, and monitoring upper limb function or falls. The gathered data is seamlessly transmitted to BioDigit Cloud, ensuring secure and compliant data collection, storage and access.
BioSensics' clinical trial products have been utilized by 21 pharmaceutical companies in 25 studies in 15+ languages.