November 30, 2022
NIH Selects BioSensics to Develop Remote Measurement Technologies for Rare Diseases
BioSensics has been selected by the U.S. National Institute of Health to develop remote measurement technologies for use in clinical trials in individuals with rare diseases.
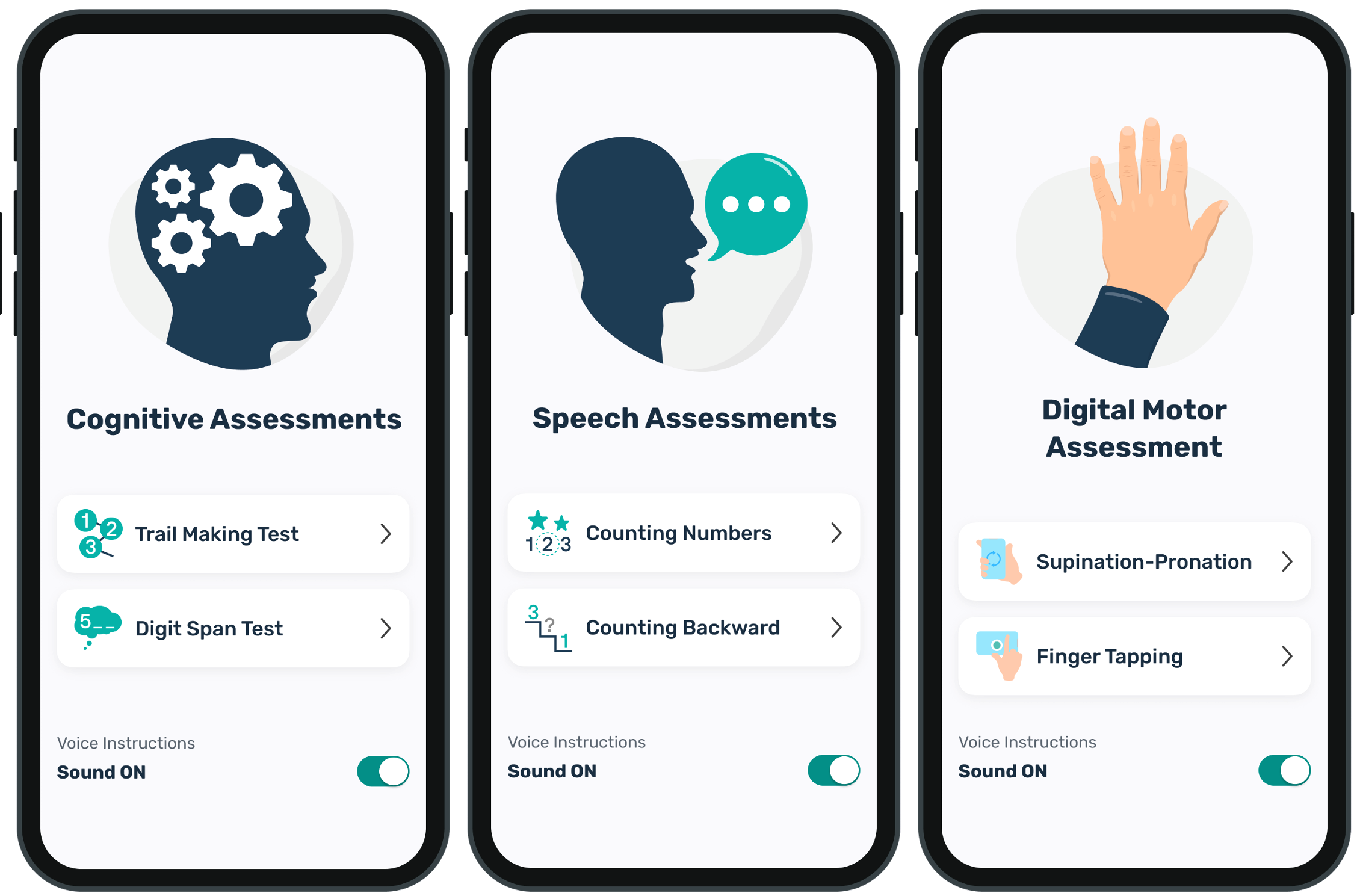
BioSensics will employ its BioDigit Mobile solution to develop robust multi-modal remote measurement and monitoring solutions for multiple rare diseases. BioDigit Mobile utilizes mobile phone sensor technologies, digitized clinical assessments, and patient reports to enable remote monitoring of motor, speech, and cognitive function, as well as mental health using smartphones.
BioDigit Mobile can be used to collect data from a large number of patients, creating unprecedented opportunities for remote monitoring of digital biomarkers and endpoints in clinical trials and research. BioDigit Mobile includes software and engagement features to simplify patient recruitment and minimize the burden on participants while improving satisfaction and compliance.
About BioSensics: BioSensics is the leader in developing wearable sensors and digital health technologies for clinical trials, remote patient monitoring, and health assessments. Founded in 2007 by three scientists from Harvard, BioSensics has created new paradigms in using wearable sensors in healthcare and revolutionized the medical alert industry by creating technologies that are now being used by thousands of older adults.
BioSensics is the only company that develops and provides end-to-end solutions and services for collection of digital measures and biomarkers in clinical trials and research. All components of BioSensics solutions, including the wearable sensors, software and algorithms are developed and validated by BioSensics. The U.S. National Institute of Health (NIH) has awarded BioSensics over $50M to support its research and development programs. In 2022, NIH selected BioSensics to develop remote measurement technologies for use in clinical trials in individuals with rare diseases.