May 18, 2022
Digital Biomarkers of ALS
There are no known cures for ALS, and only five FDA-approved drugs for addressing disease symptoms. One key challenge in the development of new medical interventions for ALS, and many other CNS diseases, is that the traditional methods of measuring disease progression are largely subjective, and require in-person visits.
As a first step towards addressing these challenges in ALS trials, BioSensics is excited to announce its comprehensive ALS Digital Biomarker Study in collaboration with Milton S. Hershey Medical Center which aims to identify links between disease progression and objectively measurable digital biomarkers.
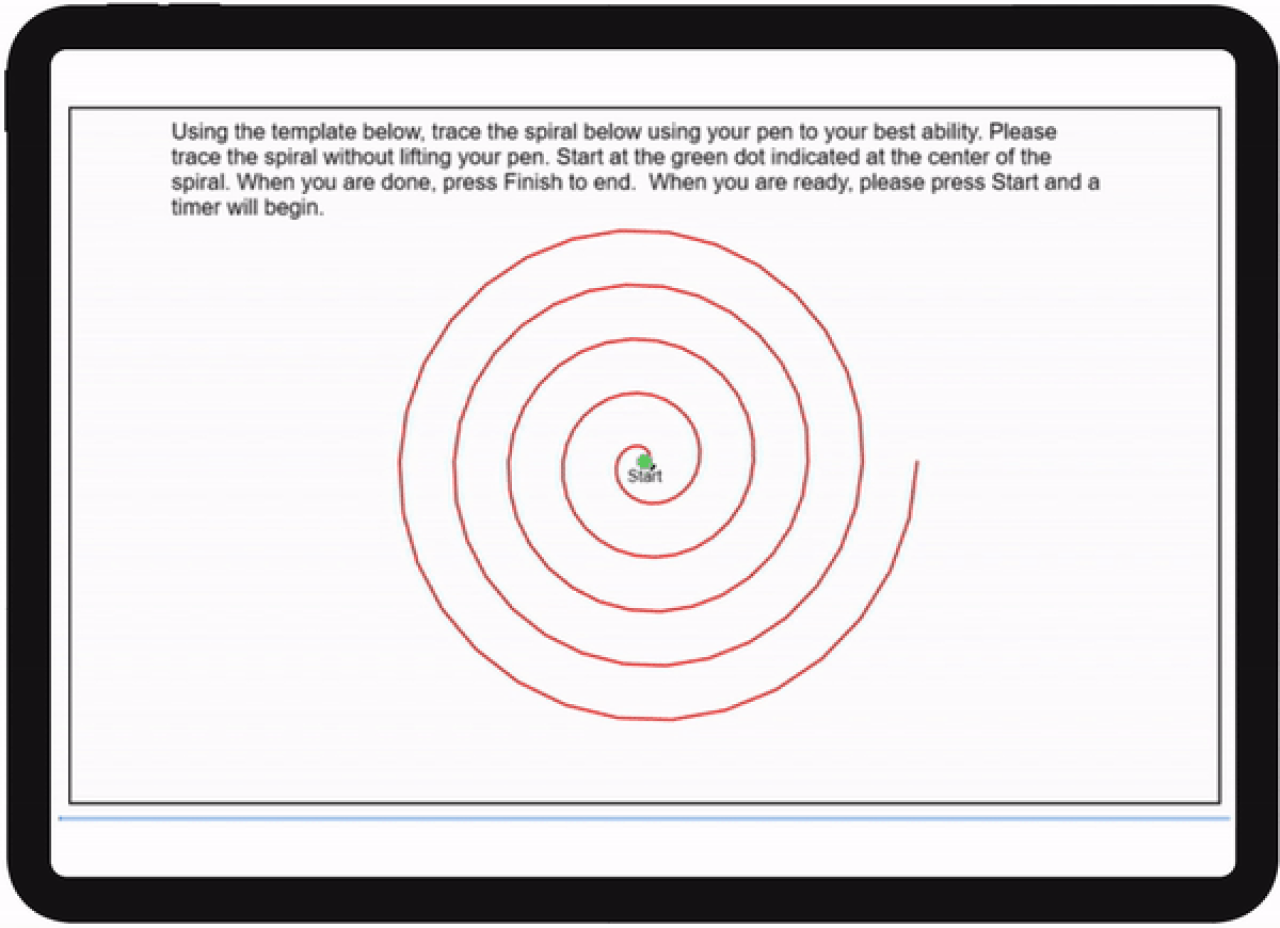
The ongoing study includes biweekly assessments of motor and speech functions, and handwriting and pattern tracing tasks at participants' homes using BioDigit Home, as well as continuous monitoring of physical activity, posture, sleep, and falls using our PAMSys sensor technology. Each participant is followed for 12 months, including 5 clinical visits.
About BioSensics: BioSensics is the leader in developing wearable sensors and digital health technologies for clinical trials, remote patient monitoring, and health assessments. Founded in 2007 by three scientists from Harvard, BioSensics has created new paradigms in using wearable sensors in healthcare and revolutionized the medical alert industry by creating technologies that are now used by thousands of older adults.
BioSensics is the only company that develops and provides end-to-end solutions and services for the collection of digital measures and biomarkers in clinical trials and research. All components of BioSensics solutions, including the wearable sensors, software, and algorithms are developed and validated by BioSensics. Our experienced research team provides comprehensive technical and scientific consulting, including study design and protocol development support, as well as statistical analysis. In addition, BioSensics clinical operations team provides comprehensive operational and logistics support for clinical trial projects.
The U.S. National Institute of Health (NIH) has awarded BioSensics over $50M to support its research and development programs. In 2022, NIH selected BioSensics to develop remote measurement technologies for use in clinical trials in individuals with rare diseases.